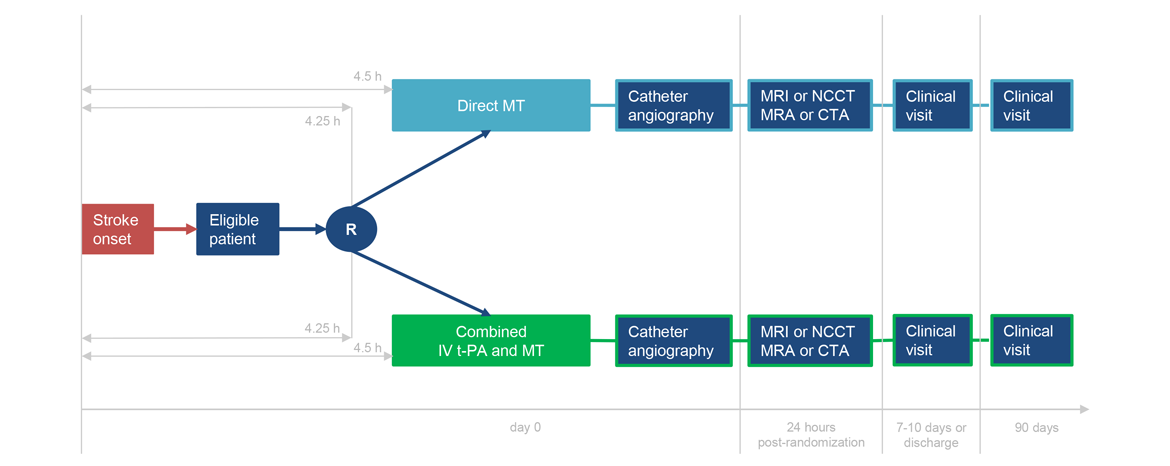
Number of randomized patients:
410
Number of recruiting sites:
48
 Key inclusion criteria (Protocol Version 3.0/4.0)
Key inclusion criteria (Protocol Version 3.0/4.0)
- Informed consent as documented by signature
- Age ≥ 18
- Acute ischemic stroke (AIS)
- NIHSS ≥ 5 and <30 (deficits judged to be clearly disabling at presentation)
- Patient is eligible for IV t-PA
- Patient is eligible for endovascular therapy
- Randomization no later than 4 hours 15 minutes after stroke symptom onset and initiation of IV t-PA must be started within 4 hours 30 minutes of stroke symptoms onset
- Occlusion (TICI 0-1) of the intracranial internal carotid artery (ICA) , M1 segment of the middle cerebral artery (MCA), or both confirmed by CT or MR angiography, accessible for MT
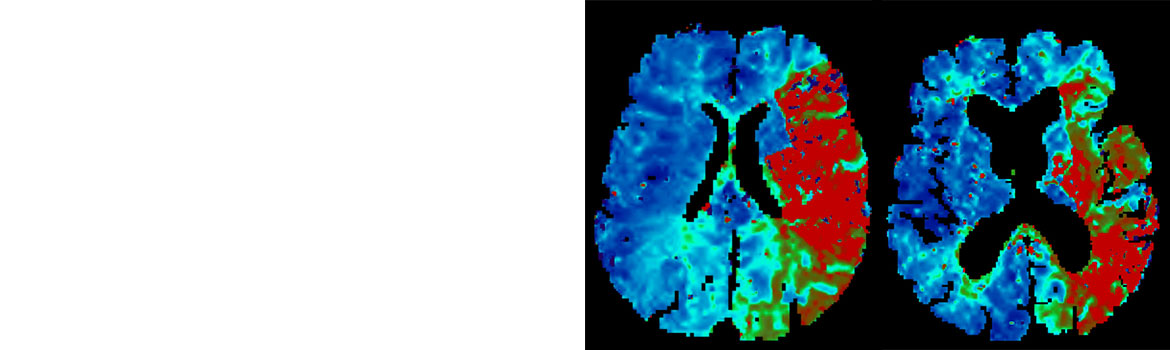
- Core-infarct volume of Alberta Stroke Program Early CT Score (ASPECTS) greater than or equal to 4 (≥ 4) based on baseline CT or MR imaging (MRI) (a region has to have diffusion abnormality in 20% or more of its volume to be considered MR-ASPECTS positive)
 Key exclusion criteria
Key exclusion criteria
- Acute intracranial hemorrhage
- Any contraindication for IV t-PA
- Pre-treatment with IV t-PA
- In-hospital stroke
- Pregnancy or lactating women
- Known (serious) sensitivity to radiographic contrast agents, nickel, titanium metals or their alloys
- Current participation in a clinical trial
- Renal insufficiency
- Life expectancy less than 90 days at baseline
- Known advanced dementia or significant pre-stroke disability (mRS score of ≥ 2)